Challenges and opportunities in SMS therapy
Elsea Lab
Baylor College of Medicine
Smith-Magenis syndrome (SMS) is caused by haploinsufficiency of the gene RAI1, which results in ~50% reduction of functional RAI1 protein. This reduced function may be caused by a deletion involving chromosome 17p11.2 (90% of cases) or due to a mutation in RAI1. In either case, reduced function of RAI1 results in the features we commonly see in SMS, including intellectual disability, early onset obesity, sleep disorder, self-injurious behaviors, and craniofacial anomalies, among other concerns. Because the amount of RAI1 is critical, it is known as a dosage-sensitive gene, which means the gene needs to be expressed at the right level at the right time and place in order to achieve normal development and function of the nervous system.
RAI1 functions as a regulator of other genes, which results in a broad impact on physiological development and function. When RAI1 isn’t working properly or not enough is present, many other genes are affected that control sleep, behavior, satiety, neurological function, and metabolism.
A variety of therapeutic approaches can be taken to address the behavioral, sleep, and metabolic concerns for individuals with SMS, including supportive and symptomatic therapies like sleep medication or physical therapy (Figure 1). Another approach is to correct or abrogate the primary genetic defect. This could be done by replacing the gene (gene therapy) or by enhancing gene expression from the remaining functional copy of RAI1 (drug therapy). The Elsea lab is taking both approaches to identify a treatment for SMS. Normalizing the expression of RAI1, while not a cure, should have a positive impact toward correcting the abnormalities observed in SMS, ultimately leading to improved quality of life.
Research update: Progress has been made with these two approaches.
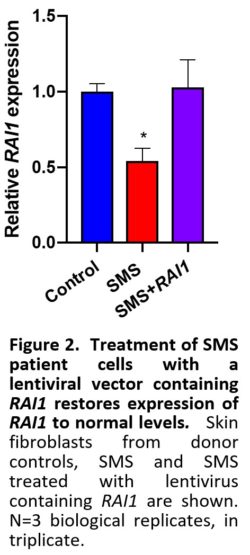
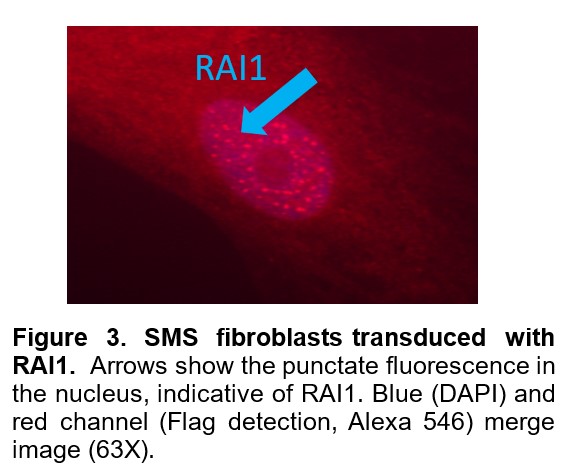
First, functional viral vectors containing RAI1 have been designed and validated. Figure 2 shows that SMS cells treated with the RAI1 lentiviral vector successfully restored levels of RAI1 to normal levelsin SMS cells. These RAI1 viral vectors are ready for injection into SMS mouse models to determine distribution and physiological impact on growth and development of the SMS mice. Figure 3 shows the RAI1 protein being expressed in these cells.
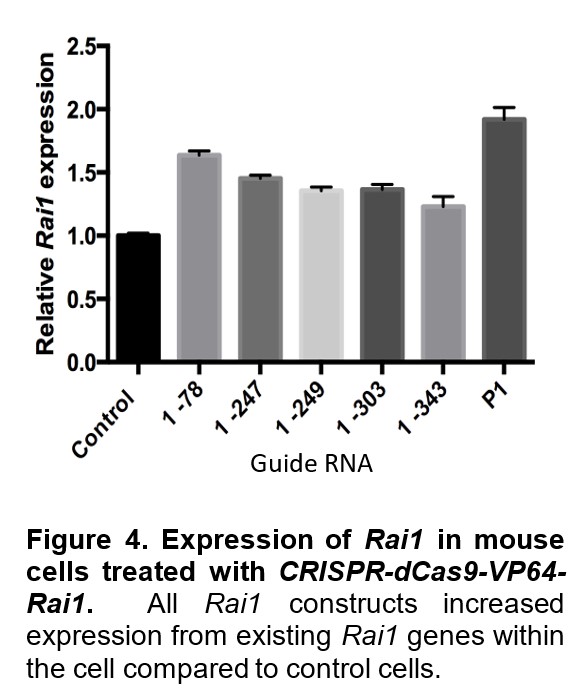
Second, we are utilizing a methodology (dCas9-VP64-guide RNA system) developed to activate the functioning, intact copy of RAI1. This system can increase the expression of RAI1 in small increments, allowing for better control of gene expression. Our approach has been successful in mouse fibroblasts. Figure 4 shows an increase in Rai1 expression in mouse skin cells treated with dCas9-VP64-guide RNA molecules mapping to different parts of Rai1. We will be injecting lentiviral particles that will deliver this activation system into mice. We are also currently designing a similar system for human cells with guide RNA that will target RAI1.
Future goals: Design and validate viral vectors 1)
containing RAI1 and 2) containing CRISPR-directed guide RNAs
for injection into SMS mice.

