Research Grant at McGill University (Extended)


Dr. Wei-Hsiang Huang’s lab at McGill University is one of the few laboratories in the world primarily focused on studying Smith-Magenis Syndrome (SMS). The Huang lab uses modern human cell lines, animal models, and cutting-edge molecular and neuroscience technologies to develop a therapy for SMS through understanding the basic neurobiology of the RAI1 protein. Since 2019, the Huang lab has published several papers that uncovered new knowledge in SMS pathogenesis and treatment. Here are some highlights:
- Uncovering the function of Rai1 in the adult mouse brain. Using a genetic trick, Rai1 was deleted from the adult mouse brain, allowing researchers to understand if Rai1 activity is required beyond embryonic brain development. Interestingly, this method uncovered that Rai1 is continuously necessary to maintain body weight homeostasis in mice by promoting the expression of a neurotrophic factor, Bdnf. Importantly, genetically enhancing Bdnf expression reverses several SMS-like features in mice. Published in Human Molecular Genetics in 2021.
- To understand how Rai1 loss regulates neuronal excitability, the Huang lab performed a large-scale brain clearing and imaging experiment and found that neurons residing in the hippocampal dentate gyrus require Rai1 to maintain activity homeostasis. Loss of Rai1 from the Emx1-lineage cortical excitatory neurons results in cortical hyperexcitability. Published in Proc Natl Acad Sci USA in 2022.
- Using a CRISPR activation system, the Huang lab developed the first gene therapy that enhances the expression of the remaining healthy Rai1 allele in the hypothalamic of SMS mice. Transcriptomic analysis, neurobehavioral assays, and metabolic measurements indicate a partial rescue of Rai1-downstream molecules and SMS-like disease features in vivo. Published in J Biol Chem. in 2023.
- Knowing the importance of Bdnf-expressing neurons and Bdnf molecular signalling, the Huang lab further studied how Rai1 regulates energy homeostasis and found that the Bdnf-expressing neurons in the PVH region depend on Rai1 for maintaining normal neuronal activity. Interestingly, a drug (LM22A-4) to enhance the neurotrophin pathway could partially rescue SMS-like disease features. In press in eLife in 2023.
- By performing comparative immunocytochemistry analyses using marmoset monkeys and mice, the Huang lab identified unique and shared patterns of RAI1 expression between these species. The lab’s work reveals the developmental and adult expression profile of RAI1 in marmoset brains and improves their understanding of the cellular basis of SMS. Published in J Comp Neurol in 2024.
With support from the SMSRF, the Huang lab is currently studying the neuroanatomical distribution of Rai1 in different species and cell types. The goal is to establish a cross-species platform to interrogate Rai1 function and test different treatments, aiming to improve the quality of life for individuals living with SMS.
2022 Research Update
 Dr. Wei-Hsiang Huang’s lab at McGill University is one of the few laboratories in the world that is primarily focused on studying Smith-Magenis Syndrome (SMS). The Huang lab uses modern human cell lines and animal models, as well as cutting-edge molecular and neuroscience technologies to develop a therapy for SMS through understanding the basic neurobiology of the RAI1 protein. Last year’s support from the SMSRF allowed Dr. Huang’s team to make preliminary, but exciting, discoveries in the function of RAI1. (Photo: SMSRF postdoctoral scholar, Dr. Lee, working through the trying time of the pandemic.)
Dr. Wei-Hsiang Huang’s lab at McGill University is one of the few laboratories in the world that is primarily focused on studying Smith-Magenis Syndrome (SMS). The Huang lab uses modern human cell lines and animal models, as well as cutting-edge molecular and neuroscience technologies to develop a therapy for SMS through understanding the basic neurobiology of the RAI1 protein. Last year’s support from the SMSRF allowed Dr. Huang’s team to make preliminary, but exciting, discoveries in the function of RAI1. (Photo: SMSRF postdoctoral scholar, Dr. Lee, working through the trying time of the pandemic.)
The second year of the SMSRF-funded project will continue this effort to develop a therapy from two angles: (1) identify druggable targets to modify RAI1 levels, and (2) reverse SMS-like phenotypes in mice by targeting an important RAI1 downstream pathway.
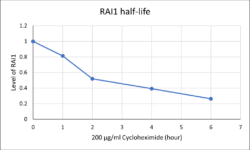 In the first year of the project, the lab’s preliminary data showed that in different human cell lines, the RAI1 protein has a very short half-life (only ~2 hours). This means that the RAI1 protein is actively being degraded by unknown pathways. While mysterious, this makes perfect sense because the RAI1 level needs to be tightly regulated in the brain: too much or too little will not work. Dr. Huang’s team is now using a small molecule inhibitor to identify druggable candidates which can regulate RAI1 stability, with the hope to finally gain control to the RAI1 protein level.
In the first year of the project, the lab’s preliminary data showed that in different human cell lines, the RAI1 protein has a very short half-life (only ~2 hours). This means that the RAI1 protein is actively being degraded by unknown pathways. While mysterious, this makes perfect sense because the RAI1 level needs to be tightly regulated in the brain: too much or too little will not work. Dr. Huang’s team is now using a small molecule inhibitor to identify druggable candidates which can regulate RAI1 stability, with the hope to finally gain control to the RAI1 protein level.
The Huang lab is currently expanding, which means more resources will be available to study RAI1 and discover disease-modifying treatments for SMS.
Grant at McGill University (Year 1)

The SMS Research Foundation (SMSRF) is excited to announce that Dr. Wei-Hsiang Huang, a leading expert in the field of Smith-Magenis Syndrome (SMS) research, has been awarded a grant in the amount of $75,000 to identify a drug to increase the level of RAI1 gene to treat SMS.
“Our Scientific Advisory Panel was very impressed with Dr. Huang’s proposal, and we feel that it complements our current gene therapy project,” said Christopher Iannuzzi, Chair of the Scientific Advisory Panel for the SMSRF.
Dr. Huang will apply modern molecular, cellular, genetic, and behavioral techniques to study how a gene imbalance impacts neural functions and causes SMS. A straightforward strategy to treat SMS is by increasing the RAI1 protein level back to normal.
“We are fortunate to be supported by the SMSRF to initiate a project to identify potential drug targets for SMS,” stated Dr. Huang. He expects this project will not only uncover multiple therapeutic entry points that warrant further pre-clinical and clinical trials, but will also provide critical insights into the basic molecular functions of the RAI1 gene.
Dr. Huang will become an Assistant Professor at the Centre for Research in Neuroscience at McGill University in the summer of 2019. The project will commence at that time. Dr Huang has been studying SMS for the past five years as a Postdoctoral Fellow in the lab of Dr. Liqun Luo at Stanford University.

